Have we misunderstood COVID-19?

To date, COVID-19 has been around for nearly a year. In 2020, the world seemed to come to a standstill, but some areas of science have been advancing at a faster rate than ever. Scientists have produced promising vaccines in 10 months, rather than 10 years. In addition, thousands of papers have been published, aiming to contribute to our understanding of the underlying mechanisms of the disease.
Initially, the scientific literature focused primarily on two main aspects of SARS-CoV-2, the virus which causes COVID-19. The first was how it binds to ACE2 receptors, which are proteins that allow the virus to hijack human cells. The second was how the infection damages the lungs. These two targets seemed to be the key to treating the disease and reducing its mortality rate. Around 1/3 of patients admitted to hospital developed acute respiratory distress syndrome (ARDS).
ARDS is a complication of several respiratory illnesses, including the flu, pneumonia, and COVID-19. However, as our experience treating COVID-19 increased, scientists noticed that the level of damage to the lungs was disproportionate to the lack of oxygen in the blood and tissues when compared to other ARDS cases. This observation led them to consider other ways that COVID-19 could damage the body.
A study published in The New England Journal of Medicine compared 7 lungs obtained from patients who died of ARDS secondary to COVID-19, with 7 lungs affected by ARDS as a complication of the flu. Alongside these, they looked at 10 uninfected lungs.
Their research showed that COVID-19 ARDS showed the typical features of classical ARDS. This included a layer of cells lining the alveoli that caused fluid to enter the lungs. In turn, this reduces the amount of oxygen getting into the blood. However, they also found features that set it apart from typical ARDS. All of which involved the endothelium, the inner lining of the blood vessels.
These features were: extensive damage to endothelial cells, an exceedingly large number of small blood clots, thickening and weakening of the endothelium, and the formation of new vessels by splitting off existing ones. This splitting is a compensatory mechanism which increases blood supply, in order to supply tissues with enough nutrients and oxygen.
Damage to endothelial cells initiates a series of reactions which leads to the formation of a blood clot. The clot is meant to stop bleeding and initiate wound healing. This damage uncovers areas and molecules, to which platelets, which form the blood clot, can be bound. As platelets continue to bind themselves to one another, they eventually form a platelet plug.
In addition, the damage setts of a coagulation cascade. The result of this process is an insoluble fibrin clot. This surrounds and stabilises the platelet plug. Healthy wound healing depends on the tight regulation of both of these processes with the participation of a vast number of molecules. The platelet plug has to be prevented from growing too big, and it eventually has to be broken down alongside the fibrin clot.
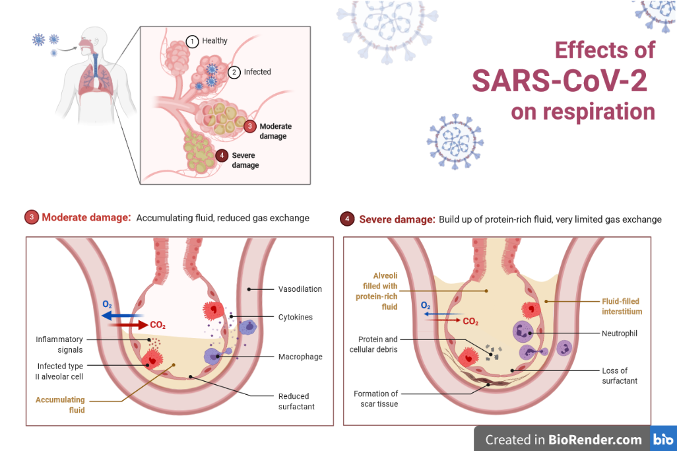
These findings tie in well with our current understanding of how SARS-CoV-2 binds to ACE2 receptors.
The ACE2 proteins can be found on the cells of the lung, the intestines, the kidney, the heart and the endothelium. Their role is to control the activity of angiotensin II. This is another protein which increases inflammation and damage to the endothelium. They do this by breaking the protein down.
When the virus binds to ACE2 receptors, it prevents them from breaking down angiotensin II. This increases inflammation and endothelial injury. Angiotensin II inhibits the secretion of the fibrinolysis inhibitor, preventing the breakdown of the blood clot.
Since then, several other studies have supported the theory that the endothelial layer plays an important role in the course of the infection. However, whether the differences in the presentation of COVID-19 ARDS is enough for it to be classified as an atypical subtype remains to be seen.
The research raises questions about COVID-19 ARDS. Can it be treated using the same parameters for mechanical ventilation as patients with classical ARDS due to the differences in the targets of damage? At the moment, the recommendation is to stick to the treatment guidelines already in place.
Understanding the underlying mechanisms of COVID-19 is paramount for developing effective treatments. By identifying the cells and molecules involved in the development of the immune response and complications like ARDS, we can target them more easily.
In this case, addressing endothelial dysfunction may lead to better patient outcomes. A study proposed statins, a common class of drugs used to treat high cholesterol, as a potential treatment endothelial dysfunction, as well as to prevent endothelial damage in patients at risk for cardiovascular disease, a risk factor for severe COVID-19.
With the rollout of the vaccine predicted to take months, who knows how many lives this new research could save? It will perhaps be a long time until science once again has the same life-saving impact for this many people. As we reach the end of a year of COVID, we can hopefully learn lessons about how quickly medicine can progress with enough scientists, funding, and determination.